Cleaner Air, The Ocean, and Global Warming
Reductions in sulfur dioxide (SO2) emissions in recent years, for […]
Date Posted:
September 16, 2016
Reductions in sulfur dioxide (SO2) emissions in recent years, for cleaner air, mainly in South East Asia due to a severe manufacturing sector slowdown and pollution policy changes (1,2) may attribute to current warming, since the phenomena called global dimming is involved.

The first policies to address air pollution in the 20th century, originated after UK’s Great Smog of 1952, which led to UK’s Clean Air Act in 1956. The aim was to shift homes’ sources of heat towards cleaner coals, electricity, and gas, it reduced the amount of smoke pollution and sulphur dioxide. Similar was the scope of the U.S. Clean Air Act in 1963.
The Clean Air Act after 50 years
Review’s noted later:
The Quality of Urban Air Review Group (1992) concluded that dramatic reductions in the concentrations of smoke and sulphur dioxide from the mid twentieth century were “brought about as a result of burning cleaner fuels, especially the use of gas; tall stacks on power stations, and their relocation outside cities; and the decline of heavy industry.” Giussani (1994) concluded that improvements in air quality after the passage of the CAA were related to activities in the industrial sector.
In the 21st century, it was the automobile that most characterised the shift in emissions, with special significance attached to the volatile organic compounds emitted within exhaust gases and from the fuel. The evidence for these changes in London is very clear as we can see a remarkable reduction in pollutants such as sulphur dioxide and black smoke.
Less improvement in nitrogen dioxide
Nitrogen dioxide forms from nitric oxide emitted from automobiles. There have been some reductions of nitric oxide emissions in the UK but chemical reactions in the atmosphere make the reduction in nitrogen dioxide less significant. Additionally, the automobile and industries using solvents have been responsible for substantial amounts of reactive organic material in London’s air. These new emissions react under sunlit conditions to promote photochemical smog characterised by high ground level ozone concentrations. Although the amount of smoke in the air has declined, a rising number of diesel vehicles has increased the amount of the very smallest particles (just a few microns across) in the air. We now realise that these small particles (PM-10 and PM- 2.5) are strongly associated with health effects of pollutants.The changes in the nature of urban air towards the end of the twentieth century were paralleled by the development of a substantial body of European Union legislation that addressed air quality under the European Directive 96/62 Air Quality Monitoring and Management and its daughter directives. More recently, air pollution has been the focus of Clean Air for Europe (CAFE). In September 2005 the European Commission proposed a streamlined clean air strategy to protect human health and the environment aiming to cut the annual number of premature deaths from air pollution-related diseases by almost 40% by 2020 (compared with the 2000 level). CAFE pays special attention to fine particulate material and ground-level ozone. It also aims to substantially reduce the area of forests and other ecosystems suffering damage from air pollutants. London has continued to take some pioneering approaches to improved air pollution. Mayor Ken Livingstone was willing to confront issues of personal freedom by promoting a system of congestion charges for vehicles accessing the central parts of the city. The charges have brought some improvements to air pollution not only by lowering the number of vehicles, but also through improved traffic flow (Beevers and Carslaw 2005). Concerns that the required increase in the number of buses would raise the concentrations of fine particulate matter appeared misplaced as newer vehicles have lower emissions.
While some improvements were made in the last decades to address smog, air pollution, there are impacts to consider when looking at the composition of the atmosphere chemistry, especially in regards to a global masking (dimming) of manmade climate change.
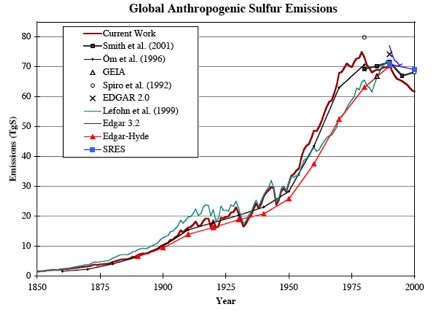
The primary cause of the mid-century cooling, through dimming, was related to the increase in atmospheric aerosols emissions.
Although the global temperature only decreased by approximately 0.1°C, this period represents a divergence from the warming periods of 1915 to 1940 and 1975 to present. (Ref)
Effects of reduced amounts of sulphur dioxide includes changes in regional rainfall patterns. To what degree the current warming may be affected by further reductions in sulphur dioxide is not quantified yet, but likely to be similar to estimates observed during the 60s and 70s.
Anthropogenic sources of sulfur dioxide decrease, but there is a theory which predicts an increase through the production by Ocean phytoplankton.
Shouldn’t we expect to find out more about the Claw hypothesis?
In 1987, Robert Jay Charlson, James Lovelock, Meinrat Andreae and Stephen G. Warren, published the study Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate (PDF), and proposed what is today known as the Claw hypothesis.
The Claw hypothesis proposes a negative feedback loop that operates between ocean ecosystems and the Earth’s climate. The hypothesis specifically proposes that particular phytoplankton that produce dimethyl sulfide are responsive to variations in climate forcing, and that these responses attempt to stabilise the temperature of the Earth’s atmosphere.
Some quotes from the 1987 study:
We begin to see a possible geophysiological link between the local self interest of salt-stress prevention and the global sulphur cycle. The accidental by-product of DMSP (Dimethylsulfoniopropionate) production is its decomposition product DMS (Dimethyl sulfide). This compound or its aerosol oxidation products will move inland from the short and deposit sulphur over the land surface downwind of the ocean. The land tends to be depleted of sulphur and the supply of this nutrient element from the ocean would increase productivity and the rate of weathering and to lead to a return flow of nutrients to the ocean ecosystems. What seems a naive altruism is in fact an unconscious self-interest.
Sulphur from DMS can travel farther than the sea-salt aerosol because several steps arc involved in the conversion of gaseous DMS to aerosol sulphate; also the resulting aerosol particles are smaller and so have much longer lifetimes. A large proportion of the current biosynthesis of DMSP is in the open oceans distant from the land surfaces. Is this DMSP also made for the relief of salt stress, or is it a redundant mechanism kept in action because of glacial epochs when the sea or part of it was saltier? Interglacials have occupied only one tenth of the time during the current series of glaciations; this may be too short a period for the devolution of DMSP biosynthesis.
Alternatively, it may be that production of DMSP in the open ocean has a different geophysiological basis from that in the continental shelf regions and one that is unconnected with salinity as such. We have seen how the local self-interest of shoreline algae could lead to the mutual sharing of sulphur and nutrients with land-based organisms. The evolution of a link between ocean climate and DMS production could have happened in a similar way. Ocean organisms are often deficient in nitrogen and to some extent vulnerable to solar ultra-violet. Cloud formation with rainfall would return nitrogen to the ocean and also serve as a sunshade. If either or both of these effects were significant for the health of phytoplankton then species that emitted DMS might be at an advantage. Is the sulphur cycle also involved in global climate control?
Evapotranspiration is known to modify the climate of forests in the humid tropics. The additional cloud cover that comes from the vast water vapour flux of the trees increases the planetary albedo and further cools the surface. The emission of DMS from the oceans seems to act similarly. Through its aerosol oxidation products it alters the properties of clouds, increasing the albedo of the oceanic regions and hence of the greater part of the planet. The link between the biota and disease in both of these processes of cloud formation could be a mechanism for climate control, the clouds serving as do white daisies in the `Daisyworid. model of Gaian climate regulation.
Lastly, DMS is the principal component of the present bio-geochemical flux of sulphur to the atmosphere. But it is not the only one; some sulphur is emitted as H2S, COS and CS2. COS, both from direct emission and as an oxidation product of CS2, is stable in the troposphere long enough to be an important source of sulphur to the stratosphere. COS in the stratosphere would be oxidized and produce a sulphate aerosol there. Such stratospheric aerosols scatter sunlight back to space and lead to a cooler climate. The biological variation of COS output is therefore another possible geophysiological means of regulating climate.
See also Cloud condensation nuclei
From a 2007 study
Phytoplankton, climate and the sulfur cycle: exploring the CLAW hypothesis (PDF)
Only a limited number of species produce high concentrations of DMSP, mainly belonging to the classes Haptophyceae and Dinophyceae (dinoflagellates), some diatoms (Bacillariophyceae) may also produce significant amounts of DMSP.
In general, it has been concluded that DMS is a product of a stressed ecosystem. The production of DMSP by phytoplankton is increased under high light and high salinity conditions, additionally, a less conclusive relationship has been proposed between DMSP production and nutrient limitation. It has also been suggested that there are two regimes of DMS production, a “bloom-driven” regime in eutrophic regions where the DMS concentration are set by phytoplankton blooms, and a “stress-driven” regime in oligotrophic open ocean regions, where DMS concentrations are highly correlated to UV radiation (Toole & Siegel, 2004). If this is the case, “stress-driven” DMS production is most likely to play a climatic role as described by the CLAW hypothesis.
One of the biggest challenges in trying to test the CLAW hypothesis is to predict global sea surface DMS concentrations accurately enough to incorporate them into coupled ocean-atmosphere climate models. The prediction of sea surface DMS concentrations and its flux to the atmosphere primarily relies on empirically-derived relationships. Several predictive algorithms have been proposed, linking sea surface DMS concentrations to biological and geophysical data, such as ocean color (used to obtain Chl a concentrations), mixed layer depth (MLD) (Simo & Dachs, 2002) and the sea surface radiation dose (SRD) (Vallina & Simo, 2007), parameters which can be relatively easily obtained by remote sensing, as well as nutrient limitation parameters (Anderson et al., 2001) derived from ocean biogeochemical models. Although these predictive algorithms have succeeded in broadly recreating the observed sea surface DMS distributions, there are errors associated with their output, up to 100% (Belviso et al., 2004) which must be taken into account. Primarily because the global database of sea surface DMS concentration observations has only limited spatial and temporal coverage, and in most cases, the predictions of these algorithms were compared against global sea surface DMS concentrations extrapolated from this data set (Kettle et al., 1999).
These different algorithms may also produce contradictory results, for instance, Anderson et al (2001) predict rather different DMS fluxes to the atmosphere for the Southern Ocean than Simo & Dachs (2002). This is because in one case DMS concentration is taken to be a function of MLD, where shallow MLDs result in enhanced DMS concentrations (Simo & Dachs, 2002), whereas this assumption is not present in the Anderson et al (2001) algorithm. In the Southern Ocean, high wind speeds result in a deep MLD, according to Simo & Dachs (2001) this would predict low DMS concentrations, however, high wind stress results in enhanced sea-air gas flux, resulting in Anderson et al (2001) predicting higher DMS fluxes to the atmosphere in this region. This brings up an interesting question as to which physical processes control DMS concentrations. The current literature strongly suggests that the surface irradiance dose is the fundamental control on DMS concentration, so a correlation between DMS and MLD is assumed because the depth of the MLD is in part controlled by surface irradiance.
Vallina et al. (2007) found that increased dust flux to the ocean and higher wind speeds in mid and low latitudes as a result of global warming would have an impact on primary production, and thus affect the production and flux of DMS to the atmosphere.
Cropp et al. (2007) developed a simple phytoplankton-DMS-cloud feedback model in order to test how the feedback might affect ecosystem stability. They found that when the model was run with the feedback, the ecosystem was more resilient to perturbations. This result addresses the question of why phytoplankton would evolve the ability to influence the properties of the atmosphere regulating the solar radiation dose. The stabilizing effects would, over the long term, favor ecosystems which generate them.
Will there be a negative response according to the Claw hypothesis with further warming, so far the data is inconclusive.
Related science
Recommendation for the differentiation of six phytoplankton groups for inclusion in future models include, eukaryotic and prokaryotic picoplankton, diatoms, dinoflagellates, and other phytoflagellates with and without DMSP-lyase activity. Emphasis is given to ecosystems dominated by the globally relevant haptophytes Emiliania huxleyi and Phaeocystis sp., which are important DMS and DMSP producers.
Photosynthetic picoplankton is especially important in the central oligotrophic regions of the world oceans, an environment that offers very low levels of nutrients.
Earth’s Oceans Show Decline In Microscopic Plant Life (2015)
Diatoms, the largest type of phytoplankton algae, have declined more than 1 percent per year from 1998 to 2012 globally, with significant losses occurring in the North Pacific, North Indian and Equatorial Indian oceans. The reduction in population may have an impact on the amount of carbon dioxide drawn out of the atmosphere and transferred to the deep ocean for long-term storage.
Phaeocystis blooms in the global ocean and their controlling mechanisms: a review (2005)
Nitzschia frigida, can modulate albedo on much the same scale as aerosols from human coal burning do, because it ranks as one of the world’s great sources of cloud-nucleating dimethyl sulfide (Ref).
The Biological Pump
The 2016 report, Explaining ocean warming: causes, scale, effects and consequences, noted:
Rising temperature reinforces Ocean Acidification. Possible feedback to global temperature from a reduced production of dimethylsulphide (DMS) by phytoplankton (Six et al., 2013).
The Biological Pump provides the main mechanism by which CO2, taken up from the atmosphere and utilized in primary production, is transferred as particulate and dissolved organic matter to the deep ocean carbon reservoir (Reid et al., 2009; Honjo et al., 2014). The remaining direct uptake by sea water of CO2 by the gas exchange pump and the solubility pump is estimated to account at present for ~10% of the transfer of carbon as dissolved inorganic carbon to the deep ocean (Honjo et al., 2014).
Temperature mediated variation in the biological uptake of CO2 by the ocean from the atmosphere has been proposed as a major factor contributing to the alternation between glacial and interglacial cycles in the Pleistocene (Matsumoto, 2007). Cool glacial temperatures strengthened the flux to the deep ocean and lowered rates of degradation reducing atmospheric concentrations of CO2. There is concern that present warming will have the opposite effect leading to increased outgassing to the atmosphere as a positive feedback.
In the Biological Pump, particulate planktonic debris and DOM (Dissolved organic carbon) produced in the euphotic zone sink to deeper depths at rates and amounts that are highly dependent on the composition of the original plankton and its associated inorganic ballast (diatom frustules, radiolarian exoskeletons) (i.e. Moigne et al., 2014).
Enroute pro- and eukaryotic organisms break down the sinking material. On reaching bathypelagic depths prokaryotic organisms predominantly, continue the breakdown and remineralization of the sinking material back to CO2, which completes the cycle by outgassing to the atmosphere in upwelling regions.
Modelling has shown that atmospheric concentrations of CO2 are highly sensitive to the depth of this remineralization (Kwon et al., 2009). The Biological Pump has a fundamental role in climate variability, and yet because of the complexity of the processes involved and their spatial, large depth and temporal variability, is still poorly quantified and understood. This complexity is illustrated by Kemp and Villareal (2013) who show that the traditional view that increased stratification reduces carbon export by a change from diatoms to small phytoplankton does not always apply.
Methane (CH4) and nitrous oxide (N2 O) are greenhouse gases that are 21 and 310 times more potent than CO2 with important sources that are affected by ocean warming (Reid et al., 2009). A potential enhanced release of a) methane from clathrates (an ice form of methane) buried in sediments on the ocean floor and from melting permafrost, and b) of nitrous oxide from expanding anoxic zones may add to the rate of global warming as the ocean heats up (Kirschke et al., 2013; Voss et al., 2013; Skarke et al., 2014).
Deoxygenation of the ocean (Keeling et al., 2010) is another consequence of ocean warming that is of high relevance to ocean productivity and marine species (Wright et al., 2012). A warming ocean reduces the solubility of oxygen in sea water, increases stability (stratification) and decreases ventilation of sea water in higher latitudes. These factors, with changes in the mixing/renewal time and the size, location and transport of water masses can reduce the transfer of oxygen below the mixed layer.
Oxygen is crucial for all aerobic marine life and is removed from sea water by the growth and respiration of the pelagic (Any water in a sea or lake that is neither close to the bottom nor near the shore), and in large part the microbial community, as it breaks down the rain of organic particles from productive upper waters as part of the Biological Pump.
Vertically migrating biota may intensify deoxygenation by the transfer of nutrients and carbon to deeper water. Extensive areas of low oxygen intermediate water between ~200 and 700m in the tropics are known as ‘oxygen minimum zones’ (OMZ). In these regions the depth of vertical migration by marine plankton closely tracks the upper margin of low oxygen waters with potential implications for the biological pump, future food webs and fisheries (Bianchi et al., 2013).
When oxygen levels drop to suboxic levels of hypoxic, is reached when levels fall below 60 μmolL-1. Only a few enclosed basins, e.g. the Black and Baltic Seas, the deep Cariaco Trench and some estuaries and fjords develop anoxic conditions where oxygen levels drop to zero (Richards and Vaccaro, 1956; Carstensen et al., 2014; Rabalais et al., 2014; Capet et al., 2016; Montes et al., 2016). There is now clear observational evidence that there has been a substantial global decline in oceanic dissolved oxygen with regions of low oxygen waters shoaling (coming closer to the surface) and expanding in their areal coverage in the last few decades (Stramma et al., 2010; Helm et al., 2011; Capet et al., 2016; Montes et al., 2016).
2015 study
Dimethylsulfide model calibration in the Barents Sea using a genetic algorithm and neural network
http://www.publish.csiro.au/?paper=EN14264
Future changes in marine biogenic aerosol emissions in Arctic seas are likely to affect the radiative budget of the region. Here we employ a calibrated biogeochemical model to simulate change in sulfate aerosol emissions in the Barents Sea, and find strong increases occur by the late 21st century. If replicated across the Arctic Ocean, such increases in sulfate aerosol loading to the Arctic atmosphere may decrease the rate of warming at polar latitudes.
The simulation results show that under tripled CO2, DMS flux would increase 168 to 279 % from autumn through winter and would increase 112 % during ice melting season. DMS flux would increase much more in ice-melt-affected water. The increased DMS flux under 3 × CO2 indicates that regional warming could slow owing to the emission of DMS in the Arctic, if the increase in emissions of anthropogenic greenhouse gases is controlled.
2016 studies
Unusual phytoplankton bloom phenology in the northern Greenland Sea during 2010
http://www.sciencedirect.com/science/article/pii/S0924796316302172
• The causes of elevated phytoplankton bloom in the northern Greenland Sea in 2010 are mainly due to the sea ice melting.
• Earlier and more extensive sea ice melt, persistent negative NAO, and changing wind directions were the main drivers of the bloom.
• Multivariate lagged regression analysis shows the bloom was correlated with the timing of sea ice melt, PAR and SST.Wind direction changed from the southeast to southwest direction in spring, possibly transporting nutrient enriched melt runoff from glaciers on Greenland and other sources from the south to northern coastal regions.
In regards to a shallow lakes
Variations in Concentrations and Fluxes of Dimethylsulfide (DMS) from the Indian Estuaries
http://link.springer.com/article/10.1007/s12237-015-0039-z
Nutrient reduction magnifies the impact of extreme weather on cyanobacterial bloom formation in large shallow Lake Taihu (China)
The frequency and intensity of cyanobacterial blooms did not significantly decrease. A total of 50.5% of the extended blooms (>300 km2) were associated with EWEs from 2007 to 2015, 36.2% of which were due to heavy rainfall and 38.3% of which were due to strong winds (25.5% were due to both). Interestingly, the frequency of the EWE-induced extended blooms significantly increased after 2012. Principal component analysis (PCA) showed that this frequency correlated positively with EWE-induced nutrient increases in the water, indicating that the complement from nutrient increases induced by EWE allow cyanobacterial cells to reach high biomass under relatively low nutrient condition. Our results suggest that EWEs play a more important role in extended bloom formation after the nutrient levels in shallow lakes are reduced.
http://www.sciencedirect.com/science/article/pii/S0043135416305619