Is Ocean Acidification an Open-Ocean Syndrome? Understanding Anthropogenic Impacts on Seawater pH
March 2013, Volume 36, Issue 2, pp 221-236 | Study | PDF | Carlos M. Duarte, Iris E. Hendriks, Tommy S. Moore, Ylva S. Olsen, Alexandra Steckbauer, Laura Ramajo, Jacob Carstensen, Julie A. Trotter, Malcolm McCulloch
Abstract
Ocean acidification due to anthropogenic CO2 emissions is a dominant driver of long-term changes in pH in the open ocean, raising concern for the future of calcifying organisms, many of which are present in coastal habitats. However, changes in pH in coastal ecosystems result from a multitude of drivers, including impacts from watershed processes, nutrient inputs, and changes in ecosystem structure and metabolism. Interaction between ocean acidification due to anthropogenic CO2emissions and the dynamic regional to local drivers of coastal ecosystems have resulted in complex regulation of pH in coastal waters. Changes in the watershed can, for example, lead to changes in alkalinity and CO2 fluxes that, together with metabolic processes and oceanic dynamics, yield high-magnitude decadal changes of up to 0.5 units in coastal pH. Metabolism results in strong diel to seasonal fluctuations in pH, with characteristic ranges of 0.3 pH units, with metabolically intense habitats exceeding this range on a daily basis. The intense variability and multiple, complex controls on pH implies that the concept of ocean acidification due to anthropogenic CO2 emissions cannot be transposed to coastal ecosystems directly. Furthermore, in coastal ecosystems, the detection of trends towards acidification is not trivial and the attribution of these changes to anthropogenic CO2emissions is even more problematic. Coastal ecosystems may show acidification or basification, depending on the balance between the invasion of coastal waters by anthropogenic CO2, watershed export of alkalinity, organic matter and CO2, and changes in the balance between primary production, respiration and calcification rates in response to changes in nutrient inputs and losses of ecosystem components. Hence, we contend that ocean acidification from anthropogenic CO2 is largely an open-ocean syndrome and that a concept of anthropogenic impacts on marine pH, which is applicable across the entire ocean, from coastal to open-ocean environments, provides a superior framework to consider the multiple components of the anthropogenic perturbation of marine pH trajectories. The concept of anthropogenic impacts on seawater pH acknowledges that a regional focus is necessary to predict future trajectories in the pH of coastal waters and points at opportunities to manage these trajectories locally to conserve coastal organisms vulnerable to ocean acidification.
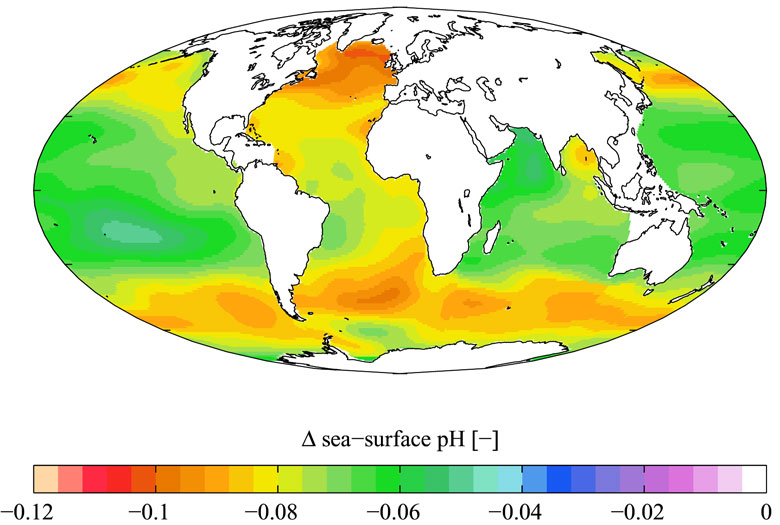
Ocean Acidification Global pH.
Study
The pH in surface open-ocean waters was regulated largely by changes in CO2 because the carbonate ion concentration (CO3 −) concentration is relatively uniform over the timescales of interest and ocean waters are mostly saturated in Ca2+ (Caldeira and Berner 1999). However, large changes occurred over longer (100 Myr) timescales (Tyrrell and Zeebe 2004). CO2 concentrations, in turn, were regulated by metabolic processes and CO2 exchanges with the atmosphere and deeper waters (Falkowski et al. 2000).
In contrast, the regulation of pH in surface coastal waters is far more complex as it depends on the processes affecting pH in the open ocean as well as watershed inputs of Ca2+, carbonate alkalinity, inorganic and organic carbon and nutrients, and ecosystem metabolism as well as hydrological processes that dictate the mixing between open ocean and coastal waters (Table 1). Watershed effects depend, in turn, on the climatic, hydrological and geological regimes as well as the dynamics of the ecosystems in the watershed (Aufdenkampe et al. 2011). Seepage of groundwater can lead to areas of high pCO2 and low pH in coastal ecosystems (Basterretxea et al. 2010). Groundwater with a low carbonate saturation state (Ω ≈ 0.5) and reduced pH (6.70–7.30) seeps through the seafloor, creating localized low seawater pH in the natural submarine groundwater springs at Puerto Morales, Mexico (Crook et al. 2012). Submarine volcanic vents or groundwater seeps can also lead to high CO2 and low pH in surface coastal waters. Volcanic CO2 vents produce shallow-water gradients of pH across tens of meters, reaching values as low as 6.6–6.8 in the Mediterranean, for example around the most active vents in Ischia and Vulcano Islands, Italy (Hall-Spencer et al. 2008; Johnson et al. 2013) and pH values <7.7 (as low as 7.21) in volcanic seeps in Papua New Guinea (Fabricius et al. 2011). Natural gradients in pH exists across a distance of <100 m from these volcanic CO2vents, where the pH increases to normal seawater values of 7.97–8.14 (Hall-Spencer et al. 2008; Johnson et al. 2013; Fabricius et al. 2011). Volcanic eruptions may lead to extreme pH, such as the 6.2 values recorded in coastal waters following an underwater eruption giving rise to a novel shallow submarine volcano south of the island of El Hierro, Canary Islands, Spain (Fraile-Nuñez et al. 2012).
Metabolic effects, represented by net community production and calcification rates, tend to be order of magnitude greater in coastal than in open-ocean ecosystems (Duarte and Cerbrian 1996; Gattuso et al.1998) and play a major role in the pH control of coastal and estuarine ecosystems (Anthony et al. 2011; Table 2). Observations of pH in a variety of coastal habitats indicate characteristic site-specific diel, semi-diurnal and stochastic patterns of varying amplitudes (Hofmann et al. 2011; Table 2). Metabolic-intense ecosystems, such as seagrass meadows, mangroves, salt marshes, coral reefs and macroalgal beds, can support diel changes in pH as high as 1.0 unit (Table 2). Coastal phytoplankton blooms can also modify water column pH significantly, with pH increasing to 8.6–9.0 during phytoplankton blooms (Brussaard et al.1996; Spilling 2007; Dai et al. 2008). Conversely, the collapse and subsequent remineralization of phytoplankton blooms can lead to substantial drops in pH, such as a seasonal decline in pH of 0.3–0.4 units reported in bottom waters at Seto Inland Sea, Japan (Taguchi and Fujiwara 2010), and Bohai Sea, China (Zhai et al. 2012).
Benthic microbial processes can also modify alkalinity and pH through sediment–water fluxes in coastal ecosystems (Cyronak et al. 2013). The primary process affecting alkalinity in anoxic sediments is bacterial sulfate reduction, which may supersaturate pore waters with CaCO3 (Berner et al. 1970; Hines et al. 1989). Net alkalinity gain is, however, only achieved during anaerobic processes that involve a permanent loss of remineralization products, e.g. through denitrification or pyrite burial (Hu and Cai 2003). Vegetation modifies sediment biogeochemical processes and, thus, affects the pH and alkalinity of pore waters. Rapid changes in subsurface biogeochemical processes measured in a New England salt marsh coincided with temporal variations in the physiology of Spartina alterniflora (Hines et al. 1989). Sulfate reduction rates increased fivefold during phases of active plant growth, probably fueled by dissolved organic matter released from the plant roots.
In summary, surface water pH in open ocean locations is relatively stable, with a narrow range of variability, typically at <0.1 pH units inter-annually (e.g. Doney et al. 2009), compared to high variability, of as much as 1 pH unit, in coastal ecosystems at scales ranging from diel (e.g. Hofmann et al. 2011; Table 2), driven by the metabolic signal to seasonal and decadal oscillations with amplitudes >0.3 pH units (e.g. Borges and Gypens 2010; Provoost et al. 2010; Barton et al. 2012; Cai et al. 2011; Hofmann et al. 2011; Mercado and Gordillo 2011; Waldbusser et al. 2011; Melzner et al. 2013; Fig. 1). In addition, as most rivers are acidic, and have saturation states for aragonite (Ω) lower than receiving ocean waters, dynamic gradients in pH and Ω exist along salinity gradients in coastal waters, which sometimes show conservative behaviour, dominated by mixing processes (e.g. Salisbury et al. 2008), but can be affected by in situ metabolic processes, deviating from conservative behaviour, in others (e.g. Cai et al. 2011).
Hence, the regulation of pH and Ω can be modelled by considering a three end-member system: open-ocean and freshwater end-members and ecosystem metabolism, the later not being properly an end-member but a process causing deviation from the open ocean to freshwater conservative mixing line (e.g. Guo et al. 2012). Ocean-dominated systems, such as the open-ocean and coastal ecosystems adjacent to arid land (e.g. those in Antarctica and adjacent to arid regions, such as NW Australia; Falter et al. 2013) and very small watersheds, such as those in atolls and small islands, will likely reflect the open-ocean pH and Ω dynamics (Falter et al. 2013). River-dominated systems will reflect the dynamics of the freshwater end-member, and the deviation of coastal ecosystems supporting intense metabolism from the conservative mixing lines delineated by the open-ocean and freshwater end-members will depend on water residence time and mixing processes (Anthony et al. 2011; Falter et al. 2013). Hence, use of coastal typologies, which consider the elements above, has led to an improved capacity to represent and integrate ocean biogeochemistry and CO2 fluxes (Laruelle et al. 2010) and may help identify contrasting modes of pH and Ω regulation in coastal waters.
Regulation of Open-Ocean and Coastal pH in the Anthropocene
The rapid increase in the capacity of humans to impact the key processes regulating the functioning of the biosphere into the Anthropocene (Steffen et al. 2007) has extended to a capacity to impact marine pH (Table 1). Human activities can act on marine pH through impacts propagated through the atmosphere, freshwater discharges and direct impacts on ecosystem components (Table 1). Accordingly, there are three main vectors of anthropogenic impacts on marine pH: (1) emissions of CO2, and other gases affecting marine pH, to the atmosphere; (2) perturbation of watershed processes affecting the inputs of nutrients, organic and inorganic carbon, acids and carbonate alkalinity to the ocean; and (3) impacts on ecosystem structure (Table 1). These drivers add to the processes operating prior to the human perturbation to regulate marine pH in the Anthropocene.
Impacts of Anthropogenic CO2 Emissions on Seawater pH
The impacts on marine pH derived from anthropogenic CO2 emissions have received the greatest attention and have led to a growing spectrum of research programs focused around the paradigm of OA by anthropogenic CO2 (Caldeira and Wickett 2003; Raven et al. 2005; Doney et al. 2009). CO2 emissions from the burning of fossil fuels and land use change since the industrial revolution have caused an increase in atmospheric CO2 concentrations from 280 to 390 ppm (globally averaged mean surface value for 2011; Thomas Conway and Pieter Tans, NOAA/ESRL, www.esrl.noaa.gov/gmd/ccgg/trends/). The global oceans have absorbed about 40 % of the anthropogenic carbon emissions (Sabine and Tanhua 2010), leading to a decline in pH evident in surface open-ocean time series (Caldeira and Wickett 2003; Raven et al. 2005; Doney et al. 2009). In addition to impacting surface water pH, accumulation of anthropogenic carbon in deeper waters is leading to shoaling of the horizon for aragonite saturation (Feely et al. 2010). Accordingly, upwelling of waters acidified by anthropogenic CO2 has led to a further decrease in surface pH, as reported in the eastern Pacific Ocean along the west coast of North America, from central Canada to northern Mexico, where shoaling of the layer of seawater undersaturated with aragonite increased the frequency and magnitude of coastal acidification associated with upwelling events (Feely et al. 2008, 2010).
Human emissions of reactive sulfur and nitrogen, derived from fossil fuel combustion and agriculture, have led to increased deposition of strong acids (HNO3 and H2SO4) and bases (NH3) to the ocean, hence affecting seawater pH (Doney et al. 2007). Whereas these effects on open-ocean pH are calculated to be minor, they can be higher, at rates of 0.02–0.12 × 10−3 pH units per year (<10 % of OA by anthropogenic CO2), in coastal ecosystems (Doney et al. 2007), where atmospheric deposition is intense and the waters can be more weakly buffered.
Impacts of Anthropogenic Watershed Perturbations on Seawater pH
Changes in land use over the past centuries have affected the biogeochemical cycles of carbon and nutrients in the coastal zone strongly (Nixon 1995; Doney 2010; Hooke and Martín-Duque 2012). In particular, human activity has altered the watershed export of organic and inorganic carbon, carbonate alkalinity, acids and nutrients to the ocean, affecting pH (Aufdenkampe et al. 2011). However, these impacts are largely restricted to the coastal ocean, where these inputs are received.
Deforestation, agricultural (Oh and Raymond 2006), mining (Brake et al. 2001; Raymond and Oh 2009) and urban/suburban practices (Barnes and Raymond 2009) were linked to direct changes in the delivery of buffering capacity to streams and rivers. These changes have the potential to alter the concentrations of inorganic C species expected through the mixing of freshwater and seawater in estuaries, thereby affecting pH in coastal water (Aufdenkampe et al. 2011). Mining activities typically yield an increase in acid export, leading to a decline in pH in the receiving coastal waters (Brake et al. 2001; Raymond and Oh 2009). In an extreme example, a pH of <3 was reported in the estuarine reaches of the Río Tinto estuary, SW Spain (Elbaz-Poulichet et al. 1999). Alteration of tropical acid sulphate soils also releases large amounts of acids (Wilson et al. 1999; Johnston et al. 2009), affecting coastal waters containing vulnerable organisms, such as corals in the inner Great Barrier Reef ecosystem (Powell and Martens 2005).
Watershed processes, including export of alkalinity, derived from the weathering of carbonate rock and the use of lime in agriculture to reduce soil acidity (West and McBride 2005) can affect the magnitude of the alkalinity buffer in coastal waters. Changes in land use and increasing precipitation and/or runoff can also enhance alkalinity export from land to coastal ecosystems through chemical weathering (Raymond et al.2008). These changes may counteract the tendency for pH to decline from OA due to anthropogenic CO2 or increase in heterotrophy from eutrophication (sensu Nixon 1995). For example, the alkalinity export from the Mississippi River to the Gulf of Mexico has increased by almost 50 % over the last 50–100 years due to increasing areas of cropland and increasing precipitation over the watershed (Raymond and Cole 2003; Raymond et al. 2008). Although freshwater discharge accounts for a large part of enhanced alkalinity export, concentrations of alkalinity in rivers have increased over time (Fig. 2a). Long-term records of river alkalinity from other areas (Fig. 2b, c) suggest a common global trend of increasing alkalinity exported from land to coastal ecosystems, which can lead to changes in pH on the order of 0.02–0.04 pH units, sufficient to offset more than a decade of OA.
Inputs of organic matter, nitrogen and phosphorus to coastal ecosystems have increased greatly (Nixon1995; Howarth et al. 1996; Conley 2000; Stedmon et al. 2006; Sharp 2010). Although eutrophication is the major concern related to these inputs, the pH of coastal waters is also influenced through the enhanced CO2uptake from primary production and CO2 release from respiration associated with increased nutrient inputs. When in balance, primary production and respiration processes result in large diel variability (Table 2), but are essentially CO2-neutral; however, over longer timescales, spatial and/or temporal decoupling of these processes can change pH drastically (Borges and Gypens 2010; Provoost et al. 2010; Cai et al. 2011). The effects of eutrophication on carbonate chemistry can exceed that of OA from anthropogenic CO2 by either increasing pH, when enhanced CO2 uptake by primary producers prevails (Borges and Gypens 2010), or by decreasing pH, where enhanced respiratory CO2 release prevails (Cai et al. 2011), a condition often associated with coastal hypoxia (Feely et al. 2010).
Impacts on pH by Anthropogenic Changes in Coastal Habitats
Coastal ecosystems contain multiple habitats that play an engineering role, affecting the physical and chemical properties of the ecosystem (Gutiérrez et al. 2011). These ecosystems include vegetated coastal habitats (seagrass meadows, macroalgal beds, salt marshes and mangroves) and coral and oyster reefs, among others (Gutiérrez et al. 2011). All coastal engineering communities support intense metabolic processes, including high primary production, respiration and calcification rates, thereby affecting CO2, CO3−, and alkalinity concentrations and surface water pH. However, many metabolically intense coastal habitats are experiencing global declines in their abundance at rates in excess of 1 % per year (Duarte et al. 2008; Ermgassen et al. 2013). These shifts in coastal habitats have major, although largely unreported, consequences for coastal pH, affecting both their mean values and variability. Likewise, the restoration and redistribution of these habitats may affect pH in coastal ecosystems significantly. For instance, Arctic warming may allow the poleward spread of macroalgae and seagrasses, which could affect the pH of the coastal waters of these highly vulnerable regions seasonally.
Current Trends in Open-Ocean and Coastal pH
Open-ocean time series show that surface ocean pH has decreased on average by 0.1 pH units since the industrial revolution (Caldeira and Wickett 2005; Orr et al. 2005; Doney et al. 2009), with open-ocean pH decreasing steadily over the last few decades at a rate of 0.0019 per year (Doney 2010). These trends match the expectations for OA driven by increasing levels of atmospheric CO2, confirming that this vector dominates the anthropogenic perturbation of pH in the open ocean. Model calculations (Orr et al. 2005) indicate that a decrease in carbonate mineral saturation states is occurring throughout the global open ocean and will impact the polar oceans first (Orr et al. 2005). During the summer of 2008, the Canada Basin of the Arctic Ocean was undersaturated with respect to aragonite (Yamamoto-Kawai et al. 2009).
However, observations of reported trends in surface water pH in coastal ecosystems reveal a variety of patterns, including periods of both increasing and decreasing pH (Fig. 3), in response to human alterations of the biogeochemical cycles in coastal ecosystems and their watersheds. A caveat in the interpretation of long-term trends in pH in coastal systems is that the measurement scale for pH is often not reported and that conventional measurement with glass electrodes along salinity gradients may introduce uncertainties (Provoost et al. 2010). Changes in salinity of 10 units can contribute an error in these pH determinations of about 0.03 units (Easley and Byrne, 2012), and the associated uncertainty in pH measured with an electrode calibrated at a single pH is ±0.3 units over a 3-unit change in pH (Easley and Byrne 2012; Waters 2012), indicative of ±0.03 units over the 0.3-unit range typically observed in coastal systems. Collectively, these factors may introduce a random error of ±0.1, generally much less than the inter-annual range of changes observed in coastal ecosystems (typically > 0.3). As the patterns in coastal pH are based on averages over hundreds to thousands of measurements, the trends are nevertheless robust despite these uncertainties (Provoost et al. 2010).
Contrary to the reported trends in open-ocean pH, none of the available records of long-term pH change in coastal ecosystems, that we are aware of, show the decline expected from OA alone (Provoost et al. 2010; Fig. 3). Long-term changes in coastal pH display patterns driven by a much more complex suite of anthropogenic impacts than those in the open ocean. In Chesapeake Bay, for example, pH in surface waters has not displayed a clear trend over the last 60 years, although it showed high variability before 1980 (Fig. 3a). This behaviour is presumably a combination of pH increases in the mesohaline mainstem and decreases in the polyhaline mainstem of the bay (Waldbusser et al. 2011). In the Susquehanna catchment, which delivers more than half of the freshwater input to Chesapeake Bay (Schubel and Pritchard 1986), reduced coal mining activity during the last century has led to increased river pH by 0.8 units (Raymond and Oh 2009), which, together with eutrophication, has partially counteracted processes that would reduce pH in the surface layer, such as OA and increased respiration.
The pH in the Danish Straits has also increased up to the mid-1980s (Fig. 3b), but then decreased by about 0.3 units in the surface layer over the last two and a half decades. This decrease is much steeper than can be explained by OA. Nutrient reduction plans, implemented since 1987, have reduced nitrogen inputs by 50 % (Carstensen et al. 2006), but primary production remains high (Carstensen et al. 2011). The increase in surface pH before ca. 1985 is consistent with the trend in nitrogen inputs (Conley et al. 2009), whereas the subsequent decrease can be explained by enhanced ecosystem respiration.
Similar trends were observed in the southern North Sea. The pH values peaked around 1985, but declined thereafter by almost 0.4 units (Fig. 3c). This decline is far greater than can be explained by OA from anthropogenic CO2 alone, but oligotrophication was suggested as a potential reason (Provoost et al. 2010). Nutrient inputs to the area were reduced significantly and have reached levels similar to those in the early 1970s, but phytoplankton biomass and, hence, primary production have not responded accordingly (Duarte et al. 2009; Carstensen et al. 2011). Thus, the large decline in pH could be due to a combination of OA, oligotrophication and enhanced respiration.
Conversely, pH in Tampa Bay displayed a somewhat different pattern, with strong increases up to 1980 followed by an almost instantaneous drop in pH and then a general increasing trend (Fig. 3d). Population growth in the Tampa Bay watershed has been high during this period, and nutrient removal from wastewater was not initiated until 1980 (Greening and Janicki 2006). A drop in primary production following this removal could explain the large decline in pH shortly thereafter throughout the bay. Seagrasses have expanded and water quality improved after implementation of the nutrient management plan, which should lead to enhanced CO2 uptake and pH increase.
Remarkably, these multi-decadal trends in coastal pH involve fluctuations of about 0.5 pH units, much larger than the 0.1-unit decline of pH attributable to OA alone. Hence, we conclude that OA from anthropogenic CO2is, to date, a relatively minor component of pH fluctuations in many coastal ecosystems, where enhanced primary production or respiration is often the primary driver. Moreover, changes could have been even larger, but competing watershed processes have buffered many of these changes.
Future Trajectories of Open-Ocean and Coastal pH
Scenarios of OA by anthropogenic CO2, driven by CGMs, predict a decline of pH by 0.3 units and a shoaling of the horizon for dissolution of carbonate minerals, particularly aragonite, by the end of the century as a result of increased anthropogenic CO2 in the ocean (Caldeira and Wickett 2003, 2005; Orr et al. 2005; Raven et al. 2005; Meehl et al. 2007). Model projections (McNeil and Matear 2008) predict that winters in the Southern Ocean will be characterized by undersaturated waters with respect to aragonite by around 2040. This projection is consistent with the models (Orr et al. 2005) which predict that the Southern Ocean surface waters will be undersaturated in aragonite throughout the year by 2050.
Upwelling waters are corrosive naturally, but their pH may decline further over time due to OA. Accordingly, the pH of coastal waters affected by coastal upwelling is expected to decline in the future. A modelled simulation of the California Current upwelling region (Gruber et al. 2012) forecasts that summer-long undersaturation will occur in the top 60 m of the water column by year 2040 and that by 2050 aragonite saturation states greater than 1.5 will have disappeared, driving more than one half of the water column to undersaturation year-round. Additionally, the seafloor will be undersaturated year-round within the next 20–30 years.
However, global model projections have coarse resolution, with grid cell sizes of 200 × 200 km or more, reflecting limitations of the ocean GCM component of global coupled climate and ocean circulation–biogeochemical models. Although models of improved resolution for ocean circulation are being developed, they do not yet incorporate biogeochemical properties. Consequently, models offering projections of future ocean pH and the saturation state of carbonate minerals only resolve adequately the open ocean and thus are incapable of resolving even the largest coastal ecosystems. Hence, the pH dynamics of coastal ecosystems are not captured adequately by current models projecting changes through the twenty-first century.
Attempts to extend current global models to coastal ecosystems may yield spurious results, unless these models capture other relevant processes, such as regional watershed processes and changes in landscapes at the ecosystem level, which are not tractable at the global scale. The difficulties in modelling pH changes in coastal ecosystems result not only from the resolution of global models but also from the greater complexity of pH regulation and the multiple vectors of anthropogenic impacts in pH operating in coastal ecosystems (Borges and Gypens 2010; Provoost et al. 2010; Barton et al. 2012; Cai et al. 2011; Hofmann et al. 2011; Mercado and Gordillo 2011; Waldbusser et al. 2011). Thus, predictions of future trajectories of pH in coastal ecosystems are still highly uncertain even though model predictions can provide reliable predictions for the future trajectories of open-ocean pH and, thereby, the open-ocean end-member affecting coastal pH. Moreover, we argue that even the expectation that the component of coastal pH change associated with OA from anthropogenic CO2 will follow the same pattern as that in the open ocean is not necessarily supported. The reason for this conclusion is that, unlike the open ocean, pCO2 in coastal ecosystems is not necessarily in equilibrium with the atmosphere at even annual timescales and many coastal ecosystems emit CO2 into the atmosphere (Laruelle et al. 2010; Cai 2011). Calculations to resolve the anthropogenic component of CO2are remarkably difficult for the coastal ocean, where the assumptions of the various methods (Sabine and Tanhua 2010) are not met, thereby precluding a direct calculation of the effect of anthropogenic CO2 on observed trends in coastal pH. Calculations based on mixing between an open-ocean end-member displaying the trajectories predicted from OA and the freshwater end-member are also unreliable because pH and the carbon system do not necessarily behave conservatively within the coastal zone and because the freshwater end-member may also shift into the future.
Hence, paradoxically, we lack guidance on the future trajectories of pH in coastal ecosystems where some of the most vulnerable taxa to OA live (e.g. Doney et al. 2010; Hendriks et al. 2010a; Kroeker et al. 2010). Producing these forecasts requires the coupling of scenarios of global CO2 emissions with regional scenarios of likely changes in the inputs of inorganic and organic carbon, nutrients, acid and carbonate alkalinity from watersheds, and trajectories of habitat changes, thereby encompassing all relevant anthropogenic drivers of marine pH change. Coupling ocean and riverine biogeochemistry models is a first, necessary step, if a challenging one (Aufdenkampe et al. 2011). Nested models may help generate future scenarios for pH in coastal ecosystems at the regional level. These models would be composed of regional oceanographic modelling systems, forced both by GCMs, capturing the transport of anthropogenic CO2 to the coastal ocean and watershed processes. Integrating all changes that may occur on a watershed with the potential to impact coastal pH downstream (e.g. changes in land use, nutrient export, changes in runoff, changes in industrial and urban exports of alkalinity, mining activities) over a century timescale is challenging.
Depending on the balance between the anthropogenic drivers impacting marine pH, the trajectories of future pH of some coastal ecosystems will range from steep acidification, several-fold faster than expected from anthropogenic CO2 emissions alone, to basification. Coastal ecosystems with attenuated or low-level watershed influences, such as Antarctic ecosystems and those adjacent to arid regions, are expected to show patterns consistent with OA by anthropogenic CO2 as they typically show little pH variability comparable to open-ocean waters (Hofmann et al. 2011; Matson et al. 2011; Falter et al. 2013). In general, increasing nutrient inputs, as expected throughout much of the developing world (Nixon 2009), may lead to increased pH, whereas oligotrophication (Nixon 2009) may lead to acidification, adding to the same trend imposed by anthropogenic CO2 emission. Loss of vegetated coastal habitats should lead to a decline in pH, whilst loss in the cover of corals and oyster reefs and regime shifts towards a great dominance of macroalgae may lead to increased pH (Anthony et al. 2011). The intensification of the hydrological cycle, with increased freshwater discharge, may also lead to decreased pH, although this effect may be partially compensated by increased alkalinity export, depending on land use changes. This change is likely to be most dramatic in Arctic coastal waters, which are rapidly freshening due to the melting of ice on glaciers and permafrost (McPhee et al. 2009), accelerating OA and Ωaragonite decline relative to the rates expected from anthropogenic CO2 alone (Tank et al. 2012). Freshening of the Arctic is so intense (McPhee et al. 2009) that the Arctic Ocean, progressively transformed into the Arctic Ocean Estuary (McClelland et al. 2012), is possibly the only ocean basin where OA by anthropogenic CO2 may not suffice to account for the observed and predicted pH and Ωaragonite trends.
Related
Chemical weathering is an integral part of both the rock and carbon cycles and is being affected by changes in land use, particularly as a result of agricultural practices such as tilling, mineral fertilization, or liming to adjust soil pH. These human activities have already altered the terrestrial chemical cycles and land-ocean flux of major elements, although the extent remains difficult to quantify. When deployed on a grand scale, Enhanced Weathering (a form of mineral fertilization), the application of finely ground minerals over the land surface, could be used to remove CO2 from the atmosphere. The release of cations during the dissolution of such silicate minerals would convert dissolved CO2 to bicarbonate, increasing the alkalinity and pH of natural waters. Some products of mineral dissolution would precipitate in soils or be taken up by ecosystems, but a significant portion would be transported to the coastal zone and the open ocean, where the increase in alkalinity would partially counteract “ocean acidification” associated with the current marked increase in atmospheric CO2.
Other elements released during this mineral dissolution, like Si, P, or K, could stimulate biological productivity, further helping to remove CO2 from the atmosphere. On land, the terrestrial carbon pool would likely increase in response to Enhanced Weathering in areas where ecosystem growth rates are currently limited by one of the nutrients that would be released during mineral dissolution. In the ocean, the biological carbon pumps (which export organic matter and CaCO3 to the deep ocean)may be altered by the resulting influx of nutrients and alkalinity to the ocean. This review merges current interdisciplinary knowledge about Enhanced Weathering, the processes involved, and the applicability aswell as some of the consequences and risks of applying the method.
About the Author: CLIMATE STATE
POPULAR
COMMENTS
- The risk with the path to a hothouse Earth | Climate State on Climate Tipping Points Existential Threat to Our Life Support Systems
- Robert Schreib on Electricity generation prices may increase by as much as 50% if only based on coal and gas
- Robert Schreib on China made a historic commitment to reduce its emissions of greenhouse gases
- Lee Nikki on COP30: Climate Summit 2025 – Intro Climate Action Event
- Hollie Bailey on Leaders doubled down on fossil fuels after promising to reduce climate pollution